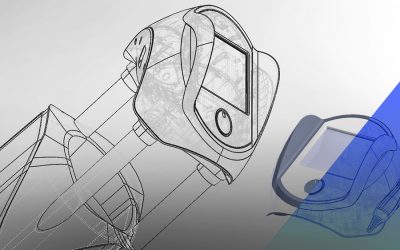
What is Medical Device Contract Manufacturing and How it helps to OEM?
What is Medical Device Contract Manufacturing? Medical Device Contract Manufacturing is a process where a MedTech company that owns the idea (sole proprietorship) outsources manufacturing to another Medical device manufacturing firm. The product is...
read moreMedical Device Manufacturing Cheat Sheet : Ways to improve Go-To-Market time
Taking a me-too product to market is easy and comparatively less hectic as you just have to find a substantial equivalent (510k) to start selling. On the other hand, a new innovation led product requires much higher investment for clinical trials,...
read more510(k) Approval Process : A Comprehensive Guide
Getting your Class II Medical Device in US Market requires several steps of 510k clearance. Before you begin the submission it’s necessary to understand, what is exactly 510k? Medical Device Manufacturers and Developers must be ready with required...
read moreMedical Device Manufacturing Services: Evolutionary Trend in 2022
The COVID -19 pandemic situation created a major stir in the medical device manufacturing services globally.
read moreJohari Digital Healthcare inaugurates the ‘Johari Center of Excellence’ in IIT Jodhpur Technology Park
Innovation in Medical Technology to Go-To-Market : Johari Digital Healthcare inaugurated the 'Johari Center of Excellence' in IIT Jodhpur Technology Park on 3rd January 2022 Most research and innovations from Academia end up in publications or are shelved,...
read moreThe Johari Yearbook 2021 : Glimpses of the Year Gone By
The year has been a roller coaster ride for us. With numerous sweet memories and bags full of learnings along the way, we are entering the new year 2022. Here we take a quick glimpse of the year gone by to revisit the learnings & joy of highlight events in...
read moreMedical Device Design and Development : The Ultimate Guide from scratch
Medical Device Design & Development is a multi-step process involving various resources and massive investments and long timelines. The process has sequential stages of ideation, designing, development, packaging, labeling, and delivery. Your Medical...
read moreMedical Device OEM: Complete Guide to Manufacturers
The medical device industry is undergoing a dynamic shift and Medical Device OEM manufacturers are focused on bringing new technologies to the market.
read moreMost Popular Wearable Healthcare Devices
Gone are the days when the winter mornings were about blankets, loaded breakfast & heavy meals. With health becoming a priority, there has been a gradual shift in the way people managed their routines. The change in the way you and I perceive health has guided market trends in the medical & healthcare industry.
read moreSoftware development in Medical Device Manufacturing: Coping with SCAM (Social, Cloud, Analytics and Mobility areas)
As a Medical Device Manufacturer, we are continually evolving capabilities to serve our clients the best products with minimal software glitches and maximum functionality relevant to the end-user.
read moreValue Engineering of Medical Devices: Busting 6 Myths to Deliver Cost Optimized Medical Device
The entire medical device manufacturing landscape is dominated by regulatory norms EU MDR 2017/745, and big investments in manufacturing, branding, and distribution. The strict regulatory norms add to the manufacturing cost at different phases of development.
read moreMedical Device Manufacturing in USA: In-Demand Medical Devices
Device Manufacturing involves multiple phases and big investments. Many public and private sector MedTech giants are investing in the sector making the market extremely competitive. With the pandemic-driven fast-paced growth of accessible healthcare devices, Medical Device manufacturers in United States along with the rest of the world are preparing strategies to cope
read moreCost Optimized Medical Device Manufacturing: Do’s and Don’ts
Medical Device Manufacturers face increasing pressure on keeping manufacturing cost-optimized. Strategic measures are required to keep the price competitive without putting quality, usability, and functionality at stake.
read moreHow MedTech companies can protect their Intellectual Property (IP) while outsourcing?
Medical Device Outsourcing by definition gives an impression of a key to unlock profit by reducing Time-To-Market, optimizing cost, and adding new ideas to the existing design
read moreWearable Device Manufacturing Company
The wearable medical device market is estimated to grow from $27.91 billion, in 2019 to $74.03 billion by 2025 at a CAGR of 17.65%, according to a report by Mordor Intelligence. The need for faster health monitoring, diagnosis,
read more