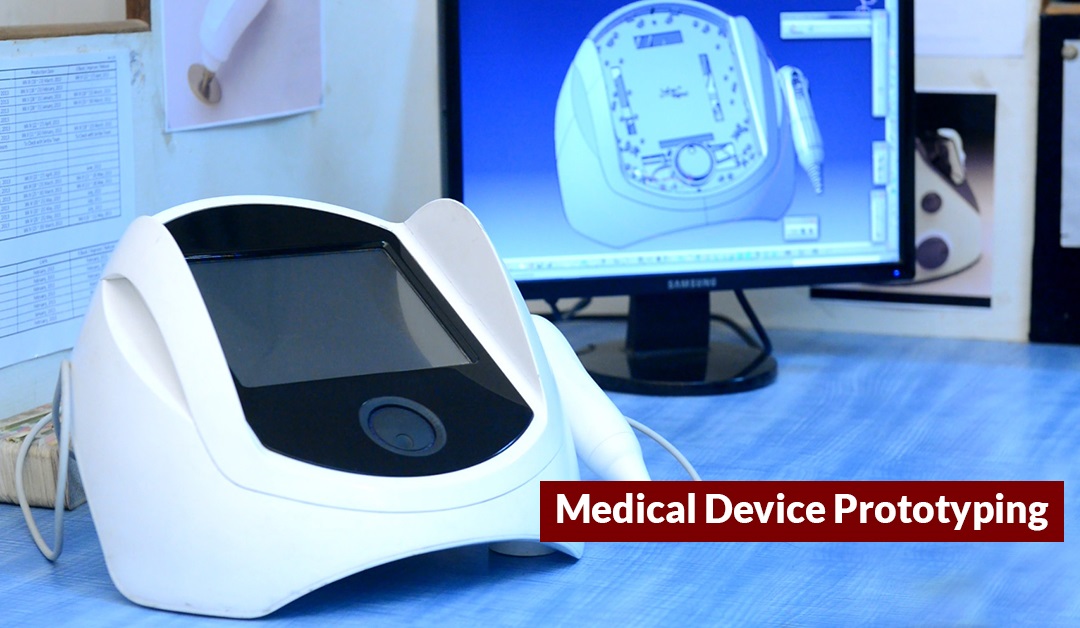
Launching & manufacturing a new medical product in the market entails multiple risks. Medical Device Prototyping is done to identify opportunities and challenges in the initial stages of medical device design development. It is necessary to evaluate end design, function, and material at the prototype level. A minor mistake or ignorance at the prototype level can complicate manufacturing, stretch the production time and, incur a significant financial loss.
What is Medical Device Prototyping ?
Medical Device Prototyping involves analyzing the medical device design on multiple parameters of efficiency in end-user environment. At the idea level the design might look revolutionary but actually holding it in the 3-D format brings actual problems to the front.
Medical Device Prototyping is a multilevel process to check the design robustness before transferring it to the mass volume production stage. At this stage, prototype engineers can use their knowledge to make multiple iterations of the product design. The hit and trial are effective in choosing the best prototype to transfer.
Understanding Stages of Medical Device Prototyping
Medical Device Prototype Development involves four stages in general:
- Alpha Prototyping
- Beta Prototyping
- Pilot Prototyping
- Final Product
1. Alpha Prototyping

Alpha prototypes are non-functional and, these test the dimensions of the product design. Design dimensions must match the user intent of the device. This stage evaluates the ease of use the product will offer to the end-user.
2. Beta Prototyping
Beta prototypes are a step ahead of the predecessor prototype. These are slightly more advanced. The beta prototypes test the sturdiness of the parts or components. In case any error is encountered it is immediately brought to the notice of key stakeholders for rectification.


3. Pilot Prototyping
Pilot prototypes are necessary for soft launches and clinical trials. This is similar to the final product and require a final polishing to manufacture a product.
4. Final Product
This is when the design is ready for mass manufacturing. The final product has all the required modifications which address all challenges identified in the previous prototyping stages.

7 Benefits of Medical Device Prototyping
- Room for Modifications
- Time & Cost optimized
- Customization facility
- Communication
- Efficiency in an end-user environment
- Regulatory compliance
- Patent Overlap
1. Room for Modifications
Let’s suppose you are making a competitive product for a better acceptance rate. One way is to theoretically compare the designs and another is to hold the visualized design of your future product in a 3D model and compare it with the competing product. Which of these options do you feel is more effective? The prototype model gives better clarity on changes or enhancements required. The possibility of identifying modifications and scope of improvement is way higher in prototyping than other ways of analyzing the design feasibility.
2. Time & Cost Optimized
Every new product design is susceptible to modifications at a later stage. Rapid prototyping saves time and money involved in modifying the whole lot over a single prototype. As the issues are identified at the primary level the errors can be minimized. The error would have been transferred to the whole batch of production. Thus, the whole lot manufactured would have been useless and a waste of time and resources.
3. Customization Facility
CAD offers a wide range of flexibility. The prototype can be tweaked into various shapes and designs without changing the other components. Thus, an idea can be molded into various forms and checked for economic, industrial, and manufacturable feasibility.
4. Communication
A physical model communicates way better than a virtual design. It’s easy to communicate challenges in design, manufacturing, or concept with a 3D Model in hand.
5. Efficiency in an end-user environment
While crafting an idea in your head it might look like it’s the most useful invention. But when you make a 3D Model you can check the product in the end-user environment. Most importantly, you can evaluate whether the product serves the desired user intent.
6. Regulatory compliance
At the prototyping stage, you can also evaluate if the processes are in line with the regulatory guidelines of ISO 13485: 2016 and US-FDA. In case, the process does not comply with regulatory guidelines you can change the process or part which violates the guideline accordingly.
7. Patent Overlap
This is important when you want to manufacture a product for a competitive market. There may be several instances where you want your product identity to be unique. In the prototype stage, one can easily check and evaluate the design for overlap with similar existing technologies.
Johari’s Capabilities as World Class
Medical Device Prototyping Company
As a manufacturer of health technology equipment, products and systems, medical device prototyping is fundamental to your medical product design and development process. Creating early samples of your health technology products gives you the opportunity to test and evaluate the concept and work on further improvement.
We, as one of the world’s leading medical device prototyping companies, work with clients closely during every phase of the medical device prototype development lifecycle to ensure the end product receives regulatory approval.
Proven Methods for Developing Medical Device Prototypes
At Johari Digital, we guide you through the prototyping process to ensure your device is ready for production. Our approach involves the following stages:
- Proof-of-Concept (POC) Prototype: This initial prototype evaluates the feasibility of your product and assesses its suitability for production, while also identifying any potential risks.
- Concept Prototyping: Experience the visual and functional aspects of your device firsthand to optimize user experience. This stage allows you to get a realistic feel for how the device will look and operate.
- Form, Fit, and Function Prototype: A fully operational medical device designed to test its manufacturability and electromagnetic compatibility (EMC). This prototype can also be utilized for conducting clinical trials.
- Pre-Production Prototype: The final iteration of your product, serving as a comprehensive verification and validation model before initiating full-scale production.
Johari Digital provides the following medical device prototyping services:
CNC Machining
We employ CNC Machining of complex parts for rapid prototyping. We work with complex geometries, offering precise and accurate parts building. Our team has in-house experts, one-to-one collaboration, pro-active DFM, Competitive pricing, and fast turnaround time.
CNC Milling
CNC Turning
5-Axis CNC Machining
Precision CNC Machining
Vacuum Casting
Low cost for silicone molds
Fast process for parts building
Wide selection of materials
High precision and fine details
Excellent Surface Finish
Parts Finishing Capabilities
Final finishing involves making the product market-ready for matching the aesthetic requirements. For gaining the accurate appearance of the final product, the product must go under a post-finishing procedure. The post finishing services at Johari involve ensuring proper color, texture, gloss, and surface finish of the intricate parts. The post-finishing procedures include hand-finishing, sanding, blasting, polishing, painting, and printing. For surface finishing, we offer a wide array of procedures, including laser etching, anodizing, powder coating, metal plating, vacuum metalizing, chromate, chemical finishing, passivation, heat treatments, etc.
We hope this article helped you gain knowledge about Rapid Prototyping and its importance in medical device mass manufacturing. Let us know your feedback or explore Rapid prototyping with Johari Digital Healthcare. Drop in your message in comment section.
Complete below form to reach out to us!