What is New Product Introduction for Medical Device Development?
NPI (New Product Introduction) is the process of introducing a new product for mass volume manufacturing. The selected essential members from important teams collaborate to identify requirements and fulfil them to accelerate new product to market.
New product Introduction Process is a bridge connecting development to manufacturing journey. The core objective is minimizing risk without compromising on quality.
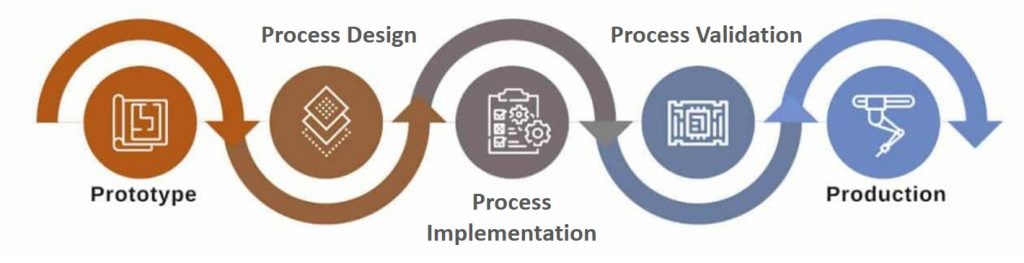
Steps involved in NPI For a Product
Step #1. Team Building
The first step of the NPI is team building. The core members involved in the project collaborate with one another to come up with best processes to scale up manufacturing of the product.
Step #2. Process Outlining
Once the team building activity is complete, specific processes involved in the process are outlined. Once the processes are in place the product is then put in the manufacturing line.
Step #3. Process Validation
Once the processes are finalized and implemented, effectiveness and impact of the processes are validated based on the products developed. Process Validation ensures compliance, consistency and quicker market reach of products.
Step #4. Continual Risk Assessment & Improvement
The Risk Assessment and improvement works continually with the entire manufacturing processes. Root cause analysis is done and improvements are made accordingly.
Sometimes, CDMO or CMO clients in Med-Tech domain have specific design modification requirements based on the market feedback. For such clients, we assist them in transforming their existing product into a more refined and successful product.
We understand the impact you want to make with your products. We keep your vision in our mind while developing the product, this helps us in delivering a product not just for our clients but also for your end-users.
New Product Introduction Process
Case:
NPI of Blood Analysis device for Sickle Cell Anemia and Malaria detection
The Microchip-Based Malaria & Sickle Cell Detection Device is curated in the Johari’s Manufacturing facility with 100% accuracy and precision. The device will fast-track the detection of fatal blood disorders.
In South Africa, Sickle Cell Anemia is one of the most pressing issues. In many regions of the country, Child mortality rate due to Sickle Cell Anemia is as high as 25% due to the absence of diagnostic centres in remote locations for quick diagnosis of this deadly disorder.
With Gazelle device, these patients can now get an accurate diagnosis in less than 15 minutes. The device is also approved as a potential gadget for Point of Care COVID-19 detection.
How do we eliminate loopholes in the New Product Introduction of a Medical Device?
Make sure processes are in synergy with continual Risk Assessment
When introducing a new product, it is important to have relevant process. Continual Risk assessment assures minimal flaws and quality products.
Check the key USPs of the Product in case of modifications
The users of previous version of the device may be satisfied with existing device. But, as a developer often you wish to add value and upgrade the existing version.
When you experiment with a product which is already a benchmark, it may fail.
Why?
Well, the product was already in good shape, offering satisfactory functionality to the end-user. When you tried making unnecessary modifications all it did is increasing price and adjusting time for the end-user.
Before experimenting, it is important to understand what you want to experiment with!
We work closely with you to understand how we can modify and help you scale without compromising the key USPs of the product.
Cross-functional coordination between teams
New Product Introduction, NPI isn’t just Product Engineering, R&D, Manufacturing, Assembly and Regulatory teams. It’s about collaborative efforts that each team is putting to take a new product to market.
We have established strategic processes for clarity in communication at each level for a quality output in the end. Clarity improves coordination and reduces unnecessary re-does and delays.
Critical tear-down analysis
A critical tear down analysis is necessary for cost incurred at every level of the manufacturing process. A clear break-up is necessary to ensure time bound management of product manufacturing.
With tear down analysis at the beginning, we make sure that you don’t have to face unpleasant surprises related to costs at a later stage.
Pre-planning to cope up with supply chain challenges
One should be ready to cope-up with supply chain challenges in the long run. Pre-planning is necessary to ensure quality throughout without hampering the production timelines.
Following 6-month Pre-plan guidelines we make sure that our clients get their consignments within the decided deadlines.
We’re the ones you’ve been looking for accelerated Go-To-Market of your Medical Devices!
With 40+ years of industry experience we help you successfully take your product to market. From complicated engineering services to mass volume production, we ace the art of New Product Introduction.
Critical analysis & sketching of the processes
We make sure that all the processes required are in sync with the specific product requirements.
Long term association with Global MEDtech’s
We’ve been working closely with Global MedTech Companies to accelerate their products successfully to the market.
Multiple Assembly lines
We have multiple assembly lines to strategically manage assembly of multiple products at once. From small hand-held products to Clinical Models, we ensure time-bound project management.
Continual Risk Analysis
Every Medical Device has a certain level of risks associated with it. Continual risk analysis from stage 1 to final commercialization stage makes sure that device offers efficient functionality as per the industry standards.
Lean Sigma Manufacturing
Eliminating unnecessary repetitive tasks with automation or any activity that promotes waste production is our main manufacturing principle. Some key aspects that we focus on eliminating while lean sigma manufacturing are defects, overproduction, waiting, non-utilized talent, transportation, inventory, motion, and extra processing.
Collaborate with us to explore seamless manufacturing services. Sign up with us to accelerate your product to market.

